Table of Contents
Introduction of organic reaction
Organic reaction is methodologies has Homolytic and heterolytic fission of covalent bonds, carbonium iron, carbanium, carbins, arynes and nitrins. mostly in organic compound molecule are atteched with covelent bond like,
ex:- R-X + Y → R-Y + X
assume here R-X and R-Y contain covalently.
- Homolytic fission – The bond between molecules connected by a covalent bond is broken in such a way that one unshared electron remains on both the resulting section.
ex:- R : X → R⋅ + ⋅X
this type of division is called Homolytic fission. it is also called free radicals. - Heterolytic fission – The bond between molecules joined by covalent bonds is broken in such a way that the electron pair of electrons remains in one section or component.
ex:- Rx ⋅ X → Rx⋅ + X⊕
if a covalent bond is dissociated during a chemical process. it is possible in two way and the intermediate is a carbonium ion or carbanion.
ex:- Rx⋅ ┋ X → R⊖⋅ + X⊕
as above if the carbon atom has a negative charge in the intermediate product [R] obtained from heterolytic fission is called carbanion.
Types and stability of carbonium ions :-
There are three types of carbonium ions. a carbonium ion has a positive charge on the carbon atom directly (1) if is attached to one carbon atom it is a primary carbonic ion (ⅰ). if it is attached two other carbon atoms, then it is a secondary carbonium ion and to another three carbon atoms if present it is called tertiary carbonium ion.
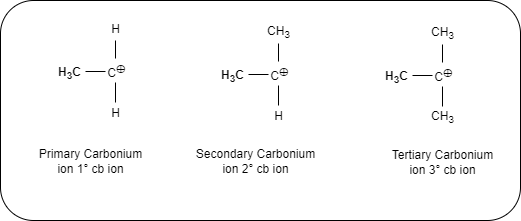
stability order of carbonium ion
⊕ ⊕ ⊕ ⊕
3° cb ion > 2° cb ion >1° cb ion > CH3° cb ion
★Reactive intermediates
- Carbenes:
⍺ – Elimination process is gives Carbene.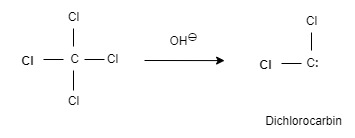
When the very strong base tertiary butoxide ion is attacked by chloroform, the carbene is formed
(ⅰ) t.BuO:⊖ + Hccl3 ⇄ :c ⋅ cl3⊖ + t.BuOH
chloroform
(ⅱ) :c.cl3⊖ → :c cl2 + cl⊖
dichlorocarbin - Arynes :
Aromatic compounds in which there is a triple bond between carbon carbon atoms.
Benzine C6H4 is the first discovered member of this that with acetylene have similarities.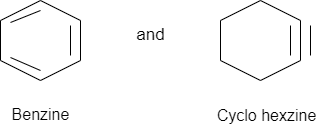
- Nitrenes :
An electron deficient covalent nitrogen species is called nitrene. In other word a nitrogen intermediates with one covalently bonded electron, with only 6(six) electron’s in its outer shell is called nitrene.
Nitrenes are formed when hydrozoic acid(HN3) is attacked by ultraviolet light.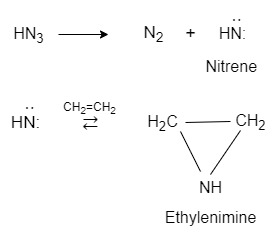
References:
https://www.masterorganicchemistry.com/reaction-guide/