Introduction of HPLC :
Chromatography is an analytical technique based on the separation of molecules due to differences in their structure and/or composition in general, chromatography involves moving a sample through the system over a stationary phase.
What is HPLC :
HPLC is an abbreviation for : High Performance Liquid Chromatography. it has also been referred to as High Pressure LC. HPLC is the largest separations technique used.
Principles of HPLC :
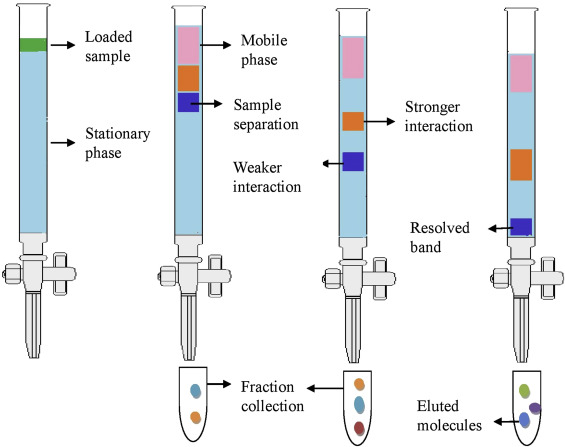
The placement (injection) of a small vol. of liquid sample onto a tube packed with porous particles (stationary phase ) where individual components of the sample are transported along the packed tube (column) by a moved by gravity.
Application of HPLC :
- Pharmaceuticals like aspirin, ibuprofen, or acetaminophen.
- Proteins like egg white or blood protein.
- Organic chemicals like polymers.
- Heavy hydrocarbons like motor oil.
- many natural products such as herbal medicines, plant extracts.
- thermally unstable compounds such as trinitrotoluene (TNT), enzyme
How Separation :
HPLC is a separation technique that involves :
- The (injection) of a small volume of liquid sample into a tube packed with tiny particles (3 to 5 micron) in diameter called the (stationary phase), where individual components of the sample are moved down the packed tube (column) with a liquid (mobile phase) forced through the column by high pressure delivered by a (pump).
- These components are separated from one another by the column packing that involves various chemical and/or physical interactions between their molecules and the packing particles.
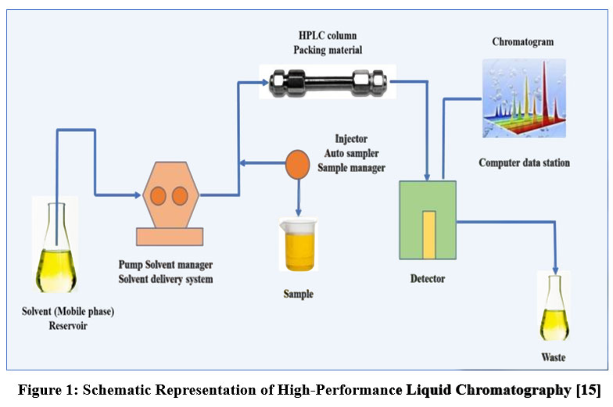
- these separated components are detected at the exit of this tube (column) by a flow-through device (detector) that measures their amount. An output from this detector is called a “liquid chromatogram”, which can be obtained by linking a computer (data processor) to the system which operates all the automatic running process.
Qualitative Analysis :
- The identification (ID) of individual compounds in the sample.
- the most common parameter for compound (ID) is its retention time.
- depending on the detector used, compound ID is also based on the chemical structure, molecular weight or same other molecular parameter.
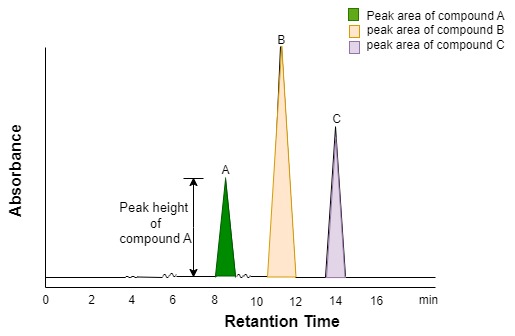
- Determination of the peak height of a chromatographic peak as measured from the baseline.
- Determination of the peak area. In order to make a quantitative assessment of the compound, a sample with a known amount of the compound of injected and its peak height or peak area is measured. In many cases, there is a linear relationship between the height or area and the amount of sample.
HPLC Analysis :
This is the chromatogram resulting from the injection of a small volume of liquid extracted from a vitamin E capsule that was dissolved in an organic solvent. modern HPLC separations usually required 10 to 30 minutes each.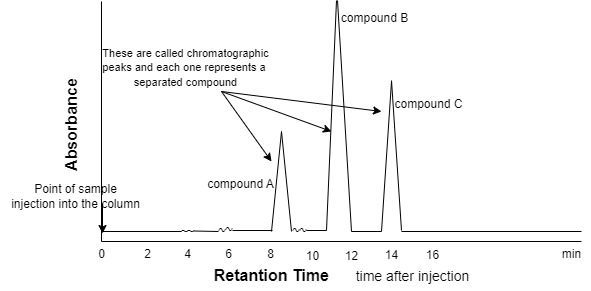
Preparation of Pure Compounds :
By collecting the chromatographic peaks at the exit of the detector, and concentrating the compound (analyte) by removing/evaporating the solvent, a pure substance can be prepared for later use (e.g. organic synthesis, clinical studies, toxicology studies, etc.)
This methodology is called preparative chromatography.
The component of HPLC :
- Pump
- Injector
- Stationary phase (column)
- Mobile Phase
- Detector
- Data Processor (Computer)
The Pump :
The role of the pump is to force a liquid (called the mobile phase) through the liquid chromatography at a specific flow rate, expressed in milliliter per min (mL/min).
Normal flow rates in HPLC are in the 1 to 2 mL/min range.
Typical pumps can reach pressures in the range of 6000-9000 psi (400-600bar).
During the chromatographic experiment, a pump can deliver a constant mobile phase composition (isocratic) or an increasing mobile phase composition (gradient).
Isocratic versus Gradient Elution:
Elution technique are methods of pumping mobile phase through a column. In the isocratic method, the composition of the mobile phase remains constant, whereas in the gradient method the composition change during the separation process.
The isocratic method is the simplest technique and should be the first choice when developing a separation.
Eluent gradient are usually generated by combining the pressurized flows from two pumps and changing their individual flow rate with an electronic controller or data system while maintaining the overall flow rate constant.
Isocratic :
Mobile phase solvent composition remains constant with time
Best for simple separation
Often used in quality control applications that support and are in close proximity to a manufacturing process
Gradient :
Mobile phase solvent (B) composition increases with time
Best for the analysis of complex samples
Often used in method development for unknown mixtures
The Injector :
The injector serves to introduce the liquid sample into the flow stream of the mobile phase.
Typical sample volume are 5 to 20 microliter.
The injector must also be able to withstand the high pressures of the liquid system.
An auto sampler is the automatic version for when the user has many samples to analyze or when manual injection is not practical.
Types of Injectors:
Manual injector :
User manually loads sample into the injector using a syringe then turns the handle to inject sample into the flowing mobile phase. which transports the sample into the beginning (head) of the column, which is at high pressure.
Autosampler :
User loads vials filled with sample solution into the auto sampler tray (100 samples).
Then auto sampler automatically measures the appropriate sample volume.
Injects the sample, then flushes the injector to be ready for the next sample, etc., until all sample vials are processed for unattended automatic operation sample.